Introduction
Transcatheter aortic valve implantation (TAVI) has become the standard for treating severe symptomatic aortic stenosis in those with prohibitive surgical risk.1 TAVI is also increasingly recommended for lower-risk patient populations.2,3 TAVI benefits include procedural success rates exceeding 95% and lower mortality than the surgical alternative.4 Delirium, an acute disorder of arousal and attention, is experienced by 23% of patients in the days post-TAVI,5 and approximately 7-14% experience cognitive decline in the following year.6
No cognitive decline or delirium risk prediction tools exist specifically for TAVI patients. General surgical and cardiothoracic risk models typically underperform in TAVI patients with specific high-risk characteristics (e.g., advanced age and multiple comorbidities).7–9 Risk prediction in frailer populations includes many general (e.g., mobility, frailty) and novel (e.g., voice, vision, and dysphagia) factors, yet these have not been applied to delirium and cognitive decline following TAVI.
This exploratory, effect-finding study investigated associations between a wide range of novel pre-procedural factors and post-TAVI delirium and cognitive decline across 12 months post-TAVI as a first step to developing applicable risk prediction models. We investigated measures spanning geriatric and frailty research and included gait, visual symptoms, voice, dysphagia, mood, and sleep.
Methods
Study population and protocol
The study population was derived from TAVI patients enrolled in a clinical trial (ACTRN12618001114235) with pre-published protocol.10 In brief, consecutive patients ≥60 years undergoing elective TAVI at the Royal Adelaide Hospital between May 2018 and May 2021 were eligible, including individuals with a diagnosed neurodegenerative condition (e.g. dementia). Eligible patients had normal hearing (with or without aid) and lived within one hour of the CBD. Exclusion criteria included current or recent (past year) alcohol or substance dependence, a diagnosed learning disability, or insufficient English language. Procedures were performed under local anaesthetic with sedation (typically propofol or dexmedetomidine). A minority received a general anaesthetic. Patients were usually mobilised 8 hours later and discharged after one night in hospital. COVID-19 restrictions from 2020 onwards severely impacted recruitment, with elective procedures cancelled from the public hospital system multiple times and moved to private hospitals (where we did not have regulatory approval). As such, we could not meet our recruitment target of 60 participants.10
We approached eligible participants at their pre-procedural consultation (typically 1-4 weeks pre-TAVI). Consenting participants completed baseline (pre-TAVI) and follow-up assessments on days 1 and 2, 3-, 6-, and 12-months post-TAVI. Trained research staff conducted assessments in participant homes or in hospital. Relevant medical history, risk scores and procedural factors were collected from hospital records. If participants could not be contacted for follow-up, we approached their next of kin.
The study was approved by the human ethics committees of the Central Adelaide Local Health Network (R20170916) and the University of South Australia (200830), and all participants provided written informed consent to participate. Participants were each reimbursed AUD$80 for the study.
Measures
The assessments we collected at which time points are given in Table 1 with details in our published protocol.10
Primary outcomes
Participants were assessed for delirium on the two days following their TAVI procedure by research staff trained by expert geriatricians (AB and DD). We used the Confusion Assessment Method (CAM)12 to ascertain delirium presence or absence, with the Memorial Delirium Assessment Scale (MDAS)13 informing CAM scoring and as a severity measure. We assigned a motor subtype using a checklist.21
We used the Addenbrooke’s Cognitive Examination III (ACE-III)11 to measure global cognition (Table 1). We classified cognitive decline at each follow-up timepoint as a decline (from baseline) of ≥3 points in ACE-III score. This cut-off was relaxed from that planned in the protocol paper (≥4) to ensure we captured all with cognitive decline and represented 1SD for healthy older adults.22 To reduce multiple comparisons, we defined cognitive decline (3-, 6- and 12-months) based on the following: (1) if only one cognitive follow-up was available, scores ≥3 points lower than baseline defined decline; (2) if two cognitive follow-ups, one ≥3 points lower and not ≥3 points lower than baseline, no overall decline was recorded (notably, this did not occur), (3) if three cognitive follow-ups, we took the majority change.
Secondary outcomes
Due to greatly reduced sample size and power, continuous cognitive change over time was not investigated as an outcome. Other secondary outcomes (quality of life, activities of daily living) will be reported in subsequent papers.
Statistical analysis
De-identified data from this trial are available by open access through the UniSA Research Data Access Portal (https://doi.org/10.25954/f1kj-w151). All data analyses were conducted using R. An F-test for numeric variables and a Chi-square test of independence for categorical variables was used to compare baseline characteristics between study completers and non-completers (those who withdrew their participation or died).
Outliers (z-score <-3 or >3) on baseline measures were removed from analyses (see Table S4 notes). We entered each factor into separate models for each primary outcome (delirium and cognitive decline), using logistic regression (odds ratos) for continuous baseline factors and Fisher’s exact tests (Cramer’s V) for categorical baseline factors. Fisher’s exact tests were not planned in the protocol but were required due to the small number of participants in dichotomous groupings.
As an exploratory, effect-finding study, we did not correct for multiple comparisons. We focused on effect sizes rather than statistical significance to identify factors with potential predictive utility for future studies.
Results
Baseline data
During the recruitment period (May 2018 to May 2021), recruitment was on hold for a total of 15 months due to withdrawal of the Chief Investigator (7 months) and COVID-19 restrictions (8 months). A total of 32 participants (20 men, mean age 82.7 years (SD 5.8)) were enrolled (Table 2). We identified mild cognitive impairment (ACE-III<8923) in 69% of the sample at baseline. Reasons for missing baseline data are provided in Table S1.
Follow-up data
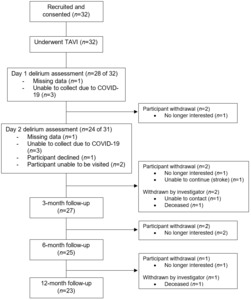
Post-TAVI follow-ups were completed by 28, 27, 25, and 23 participants at delirium assessment, 3-, 6-, and 12-month timepoints, respectively. Two participants died, and one could not be contacted. Participants also withdrew due to lack of interest (N=5) or feeling unable to continue following a stroke (N=1) (Figure 1). Non-completers had higher baseline Society of Thoracic Surgery scores, lower BMI, and fewer neuropsychiatric symptoms (Table S2).
Factors associated with delirium
Delirium was present in seven participants (25% of those assessed) (Table S3). Of these, three were hypoactive, three were hypoactive, and one had mixed delirium. The average maximum MDAS score in delirium patients was 7.4 (SD 1.5, range 5-9).
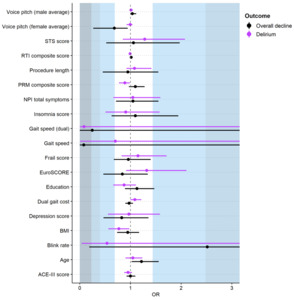
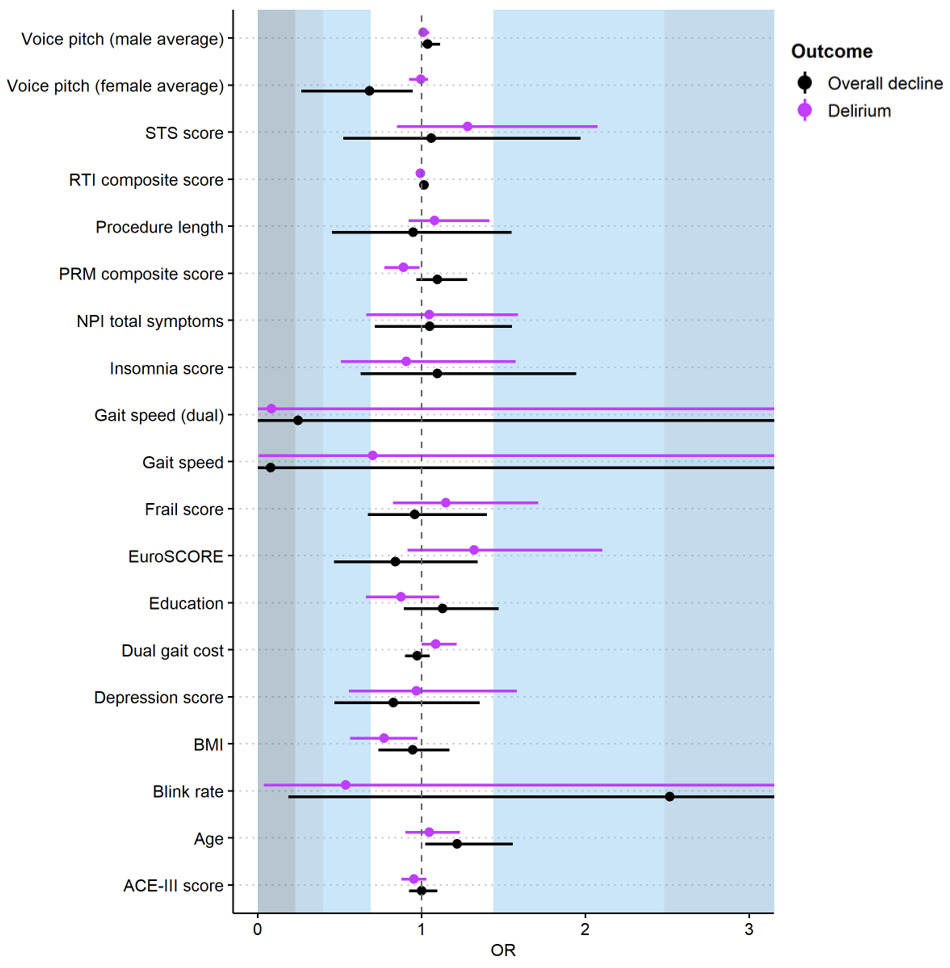
Worse baseline visual memory (Table 1) was significantly associated with delirium (OR=0.89, 95%CI 0.77 to 0.99). We found non-significant but notable associations with slower dual-task gait speed (large effect, OR=0.08, 95%CI 0.00 to 3.88), and a small effect for lower blink rate (OR=0.54, 95%CI 0.04 to 5.65) (Figure 2 and Table S4). Importantly, there was large variability around these estimates. Large but non-significant associations were found for atrial fibrillation and non-transfemoral access (both Cramer’s V=0.5). Dysphagia showed a medium effect (Cramer’s V=0.4), and weak associations were found for no renal disease, no hypertension, peripheral vascular disease, coronary artery disease, APOE-e4 allele, male gender, and more daily physical activity (Cramer’s V=0.1 to 0.3) (Figure 3, Table S5).
Factors associated with cognitive decline
We observed cognitive decline in seven participants (26% of 27 assessed, Table S6, S7). Logistic regressions did not show any significant predictors (Figure 2 and Table S4). We found non-significant associations with a large effect for slower single-task gait speed (OR=0.08, 95%CI 0.00 to 11.82), medium effects for higher blink rate (OR=2.52, 95%CI 0.19 to 35.42) and slower dual-task gait speed (OR=0.25, 95%CI 0.00 to 10.78), and small effects for lower voice pitch average (women only) (OR=0.68, 95%CI 0.27 to 0.95). Once again noting large variability around estimates indicates less confidence in these results.
Fisher’s exact tests of dichotomous predictors showed that undertaking physical activity less than daily (e.g., weekly) was significantly related to cognitive decline (strong association, Cramer’s V=0.5). Non-significant associations were found for no atrial fibrillation (medium association, Cramer’s V=0.4) and small associations for non- transfemoral access, dysphagia, no peripheral vascular disease, less daily social activity, non-APOE-e4 allele, no renal disease, no previous stroke, and coronary artery disease (Cramer’s V=0.1 to 0.3) (Figure 3 and Table S5).
Of those with delirium, only four were assessed for cognitive decline, which was positive in one case. The other three had withdrawn (N=1 had a stroke and did not wish to continue, N=2 withdrew for other reasons).
Discussion
In this prospective cohort study, 25% of TAVI patients experienced delirium and 26% cognitive decline in the year following TAVI. We identified several possible effects (although non-significant and with wide confidence intervals) for incident delirium and cognitive decline post-TAVI. Gait, dysphagia, pre-existing atrial fibrillation, and visual memory could be further investigated for delirium prediction, and physical inactivity, gait, blink rate, and atrial fibrillation for cognitive decline.
TAVI patients who partook in less than daily physical activity (pre-TAVI) were more likely to develop cognitive decline following TAVI. The recent Lancet commission identified physical inactivity as a risk factor for demenia.24 Exercise interventions can enhance cognitive function, but TAVI patients may be limited in their capacity to increase physical activity due to frailty and comorbidities.25 Even if intervention is not feasible in all cases, physical inactivity is an important risk marker which is simple to address within pre-operative risk assessment.
Poor baseline visual memory was significantly associated with post-TAVI delirium. It is unclear why the small association between poor baseline visual memory and post-TAVI delirium did not extend to global cognition or attention scores, as the overall relationship between baseline cognition and incident delirium is well-established.26–30
While not statistically significant and with wide confidence intervals, gait and dysphagia had consistent and substantial (mostly medium-large) effects for both delirium and cognitive decline. Previous research has implicated slower gait as a risk factor for dementia,31 with slowing gait shown to precede declines in cognitive function and diagnosis of mild cognitive impairment by a decade.32 Gait speed has also been implicated in TAVI, as a predictor of mortality and other clinical outcomes.33–35 Dysphagia has been overlooked in the context of TAVI, except for post-procedurally, with an incidence rate of 3%.36 Dysphagia is associated with the development of malnutrition, dehydration, and aspiration pneumonia,37 all of which can precipitate delirium.38–40 If replicated and extended in larger studies, dysphagia could be useful in preoperative risk prediction modelling for TAVI as it can be measured quickly using a single visual analogue scale.
The potential benefit of improving risk prediction in the context of TAVI would come from early intervention, detection, and diagnosis of these clinical factors. It could enable proactive decision-making by the patient and care partners or substitute decision-makers. Effective identification of those at risk is vital for delirium, considering that up to 30-40% is preventable.27,41 Furthermore, the factors we identified require minimal time, equipment, and training if found useful.
There are some important limitations to highlight. Most notably, COVID-19 restrictions impacted recruitment and sample size. Consequently, we had greatly reduced power and could not conduct all planned analyses10 or evaluate factors using multivariate modelling. We also had high attrition rates (31% by 12-month follow-up) and hence reduced ability to measure cognitive decline at later time points (6- and 12-months post-TAVI). Without a comparison group, we could not account for cognitive decline related to factors other than TAVI (e.g., age-related decline). We also acknowledge the limits of our relaxed criteria for cognitive decline (decline of ≥ 3 points on ACE-III). While ensuring all with decline are captured, our risk of Type 1 error increased. Based on our (albeit relaxed) criteria, we also saw 30% cognitive improvement in our sample. Improvements in cognition following TAVI should be considered in future research. Due to logistical difficulties in accessing participants, we could not confirm they were delirium-free immediately before their procedure. Delirium was also only assessed once daily, potentially limiting our ability to capture delirium presence. Including participants from only one public hospital site makes our sample biased towards patients within the public healthcare system, which may represent a higher-risk group.
While underpowered, our study provides some indication of novel geriatric and age-related factors that may predict delirium and cognitive decline following TAVI. TAVI patients are a unique, high-risk population who need specific risk assessment. Within this homogeneous population, negligible associations were found with typical risk factors for delirium and cognitive decline, such as increased age and depression. More importantly for TAVI patients, gait, dysphagia, pre-existing atrial fibrillation, and visual memory should be further investigated for delirium prediction and physical inactivity, gait, and blink rate for cognitive decline. With further research, incorporating these quick and simple measures could improve risk prediction for cognitive decline and delirium following TAVI and enhance targeted intervention.
Acknowledgements
We thank Erica Tilley for all her assistance with the set-up of the study. We also thank research assistants at the University of South Australia (Tyler Ross, Sara Knayfati, Charlotte Sloan) and staff at the Royal Adelaide Hospital who supported data collection throughout the trial and the participants who volunteered to participate in the research.
Author Contributions
Erica S. Ghezzi: Formal analysis, Investigation, Resources, Data Curation, Writing – Original Draft, Visualisation, Project administration. Peter J. Psaltis: Conceptualisation, Methodology, Writing – Review & Editing. Tobias Loetscher: Conceptualisation, Methodology, Writing – Review & Editing. Daniel Davis: Conceptualisation, Methodology, Resources, Writing – Review & Editing. Monique S. Boord: Software, Investigation, Resources, Writing – Review & Editing. Danielle Greaves: Investigation, Resources, Writing – Review & Editing. Joseph Montarello: Methodology, Resources, Writing – Review & Editing. Jerrett K. Lau: Methodology, Resources, Writing – Review & Editing, Sinny Delacroix: Methodology, Resources, Writing – Review & Editing. Alice Bourke: Methodology, Resources, Writing – Review & Editing. James McLoughlin: Methodology, Resources, Writing – Review & Editing. Megan Keage: Methodology, Resources, Writing – Review & Editing. Hannah A.D. Keage: Conceptualisation, Methodology, Resources, Writing – Review & Editing, Supervision, Project administration, Funding acquisition.
Ethics Statement
The Central Adelaide Local Health Network (CALHN) Human Research Ethics Committee (Reference number: R20170916) and the University of South Australia Human Ethics Committee (Reference number: 200830) approved the research with original approval obtained on 13 December 2017 and 3 April 2018, respectively. Informed consent is signed by all participants or appropriate caregiver before inclusion. Consent is continually monitored during the study by asking participants if they want to continue at the start and end of each session.
Funding sources
Professor Hannah AD Keage and Associate Professor Tobias Loetscher are supported by a NHMRC Dementia Research Leadership Fellowships (GNT1135676 and GNT1136269 respectively). Associate Professor Peter J Psaltis is supported by a Future Leader Fellowship from the National Heart Foundation of Australia (FLF100412) and an NHMRC Career Development Fellowship (CDF1161506). Professor Daniel Davis is funded by a Wellcome Trust Intermediate Clinical Fellowship (WT107467).
Declaration of Interests
None.